The electron transport chain is a series of interconnected proteins and carrier molecules that are embedded in the inner mitochondrial membrane. In order to understand the function of the electron transport chain in all its glory, we have to first appreciate two important molecules: NADH and FADH2. These two molecules can be thought of as energy dump trucks that strip sugars, fats, and proteins of their energy rich electrons (see the articles on glycolysis and the Kreb’s cycle for more details on how sugars and fats get stripped of their energy).
NADH and FADH2 then transfer their electrons to specific carrier molecules that constitute the “electron transport chain”. From there, the electrons "bounce" from carrier molecule to carrier molecule as if they were falling down an energy waterfall. Each time they bounce between carrier molecules energy is emitted; this energy is used to pump protons out of the mitochondrial matrix.
As protons get pumped out of the matrix they form an electrochemical gradient. As more electrons bounce down the chain, more protons get pumped out of the matrix. Eventually the concentration of protons outside the mitochondrial matrix is much higher than the concentration inside the matrix.
This is where the plot thickens… At this point all of the protons outside the matrix want to diffuse down their concentration gradient to their "home" in the matrix. However, the membrane that lines the matrix is impermeable to protons… Except at one specific point!
That point is a specialized enzyme embedded in the membrane called ATP synthase. The sole function of this enzyme is to rejuvenate a molecule known as adenosine triphosphate (ATP).
As the protons flow down their concentration gradient through the ATP synthase they release energy. The synthase uses this energy, via a wonderfully complicated molecular gear shifter, to form ATP from ADP (adenosine diphosphate) and free phosphate. This process is formally known as oxidative phosphorylation.
ATP is an energy rich molecule that can be thought of as the gasoline used to run a car. In simple terms, if a cell is building a molecule (ie: protein, DNA, RNA, etc.) it is likely using the energy in ATP to do it. When ATP runs out, the cell stops producing molecules and is in jeopardy of dying.
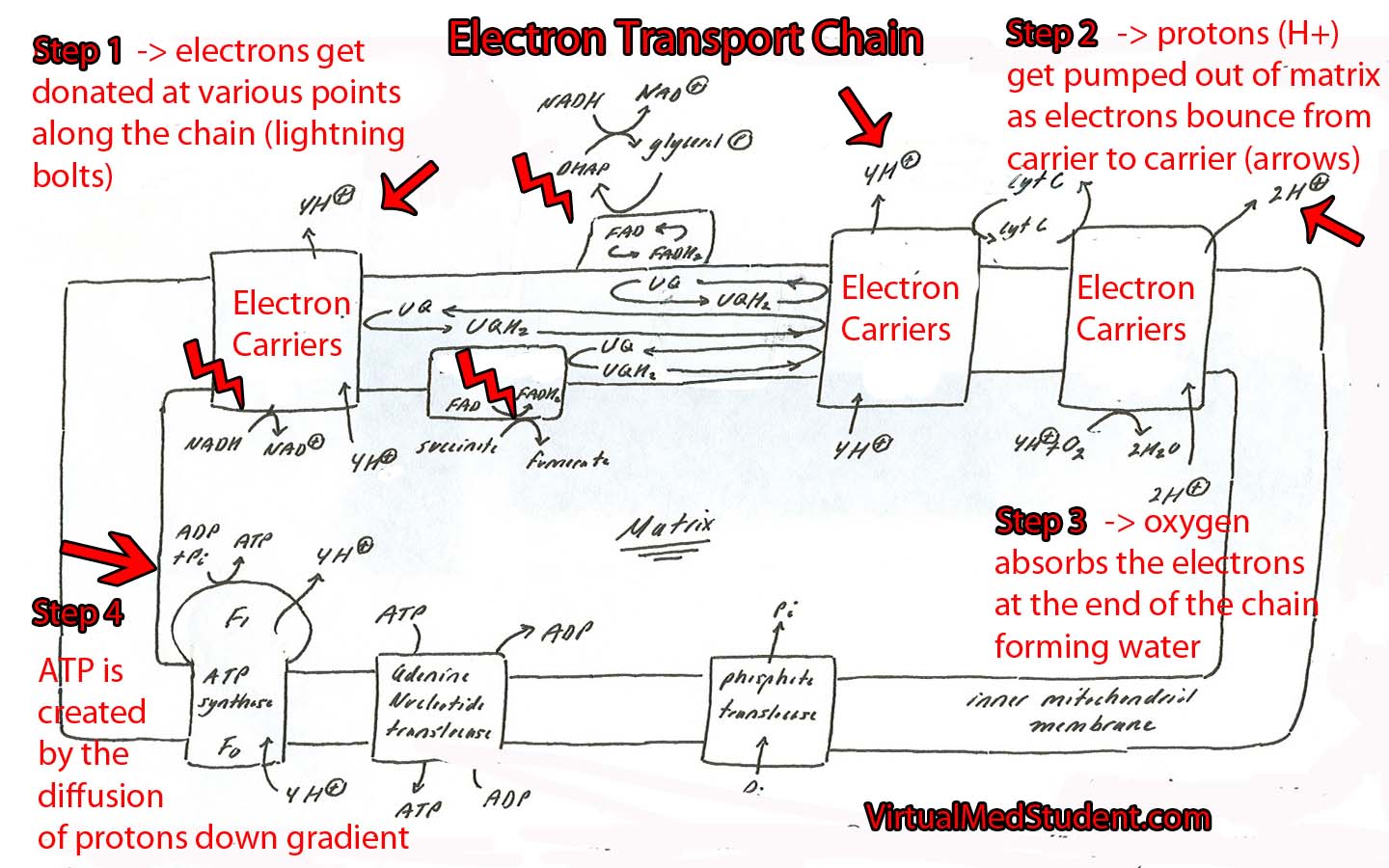
The whole point of the electron transport chain is to produce more ATP by using the energy obtained from the electron pillaging of molecules like glucose, long chain fatty acids, and proteins!
So what happens to the poor old electrons? Oxygen absorbs them at the end of the chain and forms water! This is why humans require oxygen to live! Without oxygen, electrons back up in the chain and eventually the whole transport system grinds to a halt.
Regulation
Like every other biochemical pathway in the body, the electron transport chain is under strict regulation. When ATP is abundant in the cell the chain slows down (ie: why drop electrons down the chain and strip sugars, fats, and proteins of their energy when you don’t have to?).
However, when the cell has little ATP around, then the chain springs in to action as the body burns sugars, proteins, and fats to create the electron carriers necessary to run the chain.
There are several points in the chain where things can turn awry. The first is if there is no oxygen. Oxygen is the last point in the electron "waterfall". Once the electrons get to the last carrier in the chain they have no where to go but to reduce oxygen to form water.
Without oxygen the electrons back up like lemmings. Eventually the whole chain becomes saturated with electrons and causes the whole system to stop, which can be disastrous to cellular function.
Overview
So let’s bring it all together… Cells strip sugars, proteins, and fats of their energy rich electrons. These electrons hitch a ride on special molecules like NADH and FADH2. NADH and FADH2 donate their electrons to special carrier proteins embedded in the inner mitochondrial membrane. The electrons bounce from carrier to carrier releasing energy. The released energy is used to pump protons out of the matrix. The resulting energy rich proton gradient is then used to create ATP as the protons flow down their concentration gradient back into the matrix through the ATP synthase unit… Now go drink another cup of coffee!
References and Resources
- Le T, Bhushan V, Grimm L. First Aid for the USMLE Step 1. New York: McGraw Hill, 2009.
- Kumar V, Abbas AK, Fausto N. Robbins and Cotran Pathologic Basis of Disease. Seventh Edition. Philadelphia: Elsevier Saunders, 2004.
- Champe PC. Lippincott’s Illustrated Reviews: Biochemistry. Second Edition. Lippincott-Ravens Publishers, 1992.
- Nelson DL, Cox MM. Lehninger Principles of Biochemistry. Fifth Edition. New York: Worth Publishers, 2008.
I believe, with reference to the context of free protons, the meaning is positively charged H+ ion, the remainder of glucose. Otherwise, the presence of free protons shall make glycolysis a nuclear reaction instead of a chemical one.
This is an awesome site, I will let my fellow classmates now.
Whoever made this is amazing!
wow as in wow, the explanation on how ETC process is so simple :).
This is Seriously a great site!! Straight forward explanations that make sense to a simple brain like mine!!