Cardiac muscle contracts in a predictable, regular pattern, which allows it to generate enough force to eject blood to the body. However, in ventricular fibrillation the heart muscle “quivers” in a rapid, irregular, and unsynchronized manner. These feeble contractions are not strong, nor coordinated enough to eject blood from the heart. As a result, cardiac output drops and the body quickly goes into cardiogenic shock.
There are numerous causes of ventricular fibrillation. The most common cause of abnormal electrical activity occurs in diseased heart tissue that has lost its normal architecture. For example, muscle damage from heart attacks or disorganized heart structures seen in cardiomyopathies can serve as abnormal areas of electrical impulse formation; these irritated areas can predispose patients to develop ventricular fibrillation.
Other causes of ventricular fibrillation include electrolyte abnormalities. For example, hyperkalemia (ie: an elevated blood potassium level) can depolarize heart muscle cells and make them more likely to "fire" an action potential. In general, any electrolyte disturbance that makes the resting potential of the cardiac muscle fiber more positive (ie: more depolarized) can cause abnormal electrical impulse formation; these abnormal impulses can degenerate into ventricular fibrillation.
Signs and Symptoms
Ventricular fibrillation is a highly fatal rhythm because the heart fails to pump blood, and more specifically oxygen, to the bodies’ organs. As a result, every organ system in the body, including the heart becomes ischemic and dies.
The rapid decline in blood flow to the brain causes people to lose consciousness. If treatment is not sought quickly the patient will have anoxic brain injury (ie: a massive global stroke), which will lead to brain death.
Diagnosis
Ventricular fibrillation is diagnosed by looking at an electrocardiogram (ECG). The ECG will show disorganized and chaotic electrical activity.
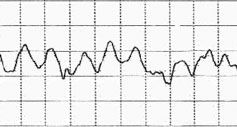
ECG of ventricular fibrillation
Treatment
Treatment of ventricular fibrillation is with immediate un-synchronized electrical cardioversion (ie: the paddle "thingies" they use to shock someone’s heart). The goal of shocking the heart with electricity is to reset (ie: repolarize) all the cardiac muscle fibers at the same time. From there the sinus node should theoretically take over, and reset the heart back into a normal rhythm.
If a patient survives their first episode of ventricular fibrillation they often have a cardiac defibrillator implanted. Implantable cardiac defibrillators shock the heart when they detect an abnormal rhythm.
Overview
Ventricular fibrillation is a rapidly fatal, disorganized, and inefficient “quivering” of heart muscle. It causes cardiogenic shock and organ death if left untreated. It is most commonly due to underlying heart disease seen in people with coronary artery disease, previous heart attacks, and cardiomyopathies, although other causes exist. Treatment is with immediate electrical cardioversion (ie: “shocking” the heart).
Related Articles
- Dilated Cardiomyopathy: Poor Pump, S3, and Crackles
- Restrictive Cardiomyopathy: Amyloid Deposits, Diastolic Dysfunction, and Kussmaul’s Sign
- Cardiac Output: Pump, Pump, Squeeze
- Atrioventricular Block: PR, QRS, and Mobitz
- The Basics of Myocardial Infarction
References and Resources
- Marcus GM, Scheinman MM, Keung E. The year in clinical cardiac electrophysiology. J Am Coll Cardiol. 2010 Aug 17;56(8):667-76.
- Dosdall DJ, Fast VG, Ideker RE. Mechanisms of defibrillation. Annu Rev Biomed Eng. 2010 Aug 15;12:233-58.
- Braunwald E. Hypertrophic cardiomyopathy: the early years. J Cardiovasc Transl Res. 2009 Dec;2(4):341-8. Epub 2009 Oct 7.
- Rea TD, Page RL. Community approaches to improve resuscitation after out-of-hospital sudden cardiac arrest. Circulation. 2010 Mar 9;121(9):1134-40.
- Schaer B, Kühne M, Koller MT, et al. Therapy with an implantable cardioverter defibrillator (ICD) in patients with coronary artery disease and dilated cardiomyopathy: benefits and disadvantages. Swiss Med Wkly. 2009 Nov 14;139(45-46):647-53.
- Callans DJ. Out-of-hospital cardiac arrest–the solution is shocking. N Engl J Med. 2004 Aug 12;351(7):632-4.
- Lilly LS, et al. Pathophysiology of Heart Disease: An Introduction to Cardiovascular Medicine. Seventh Edition. Lippincott Williams and Wilkins, 2020.