What is “hemodynamic instability”? Hemodynamics is the study of blood movement; when this movement is compromised in some way you get hemodynamic instability. If left untreated it can cause multi-organ failure and death.
You can think of hemodynamic instability as the collapse of the cardiovascular system. This collapse causes a significant drop in blood pressure.
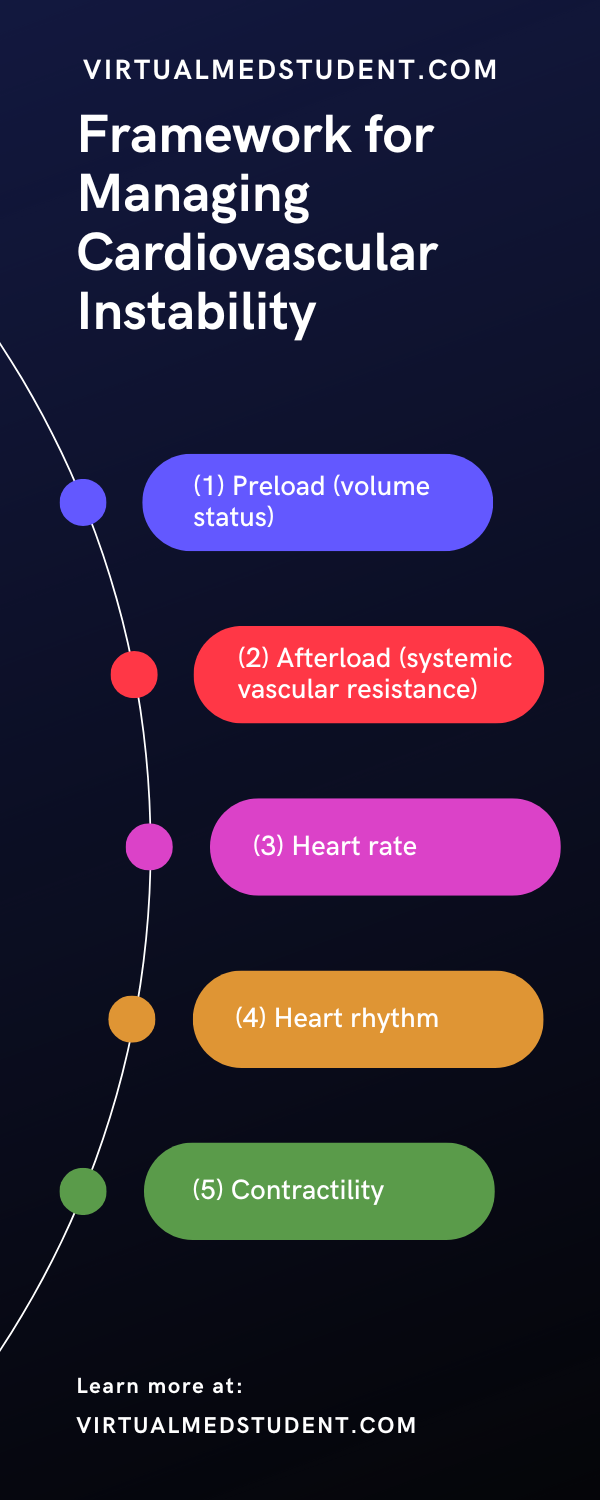
There are many causes of hemodynamic instability and fortunately you don’t need to know all of them to manage a patient who is acutely experiencing cardiovascular collapse. A few basic tenets of physiology, when examined in the right order, will help you manage a patient who is unstable.
The tenets are as follows: preload (volume status), afterload (systemic vascular resistance), heart rate, heart rhythm, and contractility.
Preload and Volume Status
The first component of cardiovascular physiology that should be assessed in a hemodynamically unstable patient is preload and intravascular volume. Intravascular volume measures how much blood is present in the circulatory system. If a patient is volume deplete (ie: has a decreased intravascular volume) then hemodynamic instability can occur. An easy way to visualize this is to imagine someone bleeding. If the bleeding is not stopped, the cardiovascular system will eventually collapse. In the hospital this is often referred to as a patient being "dry".
Preload is one way to estimate a patient’s intravascular volume. Measuring preload is easy if you have a central venous catheter, and even easier if you have a Swan Ganz catheter, although these are used less frequently nowadays. These types of catheters can measure central venous and pulmonary artery pressures, which provide a good estimate of intravascular volume status.
If a patient does not have a central line you have to rely on clinical clues. One way to assess intravascular volume is to measure urine production. If urine output is less than normal, then the patient is trying to “hold on” to fluid, and therefore may be volume deplete. Urine that is dark amber in color is another clue. Decreased skin turgor is another clinically useful sign. Examining a patient’s neck veins (ie: jugular venous pulsations) can also be useful under certain circumstances.
In essence, if a patient’s preload, and therefore intravascular volume status is decreased it can be quickly corrected by giving the patient intravenous fluids. The type of fluid given is dictated by the clinical situation, but the most commonly used fluids for hemodynamic resuscitation are normal saline, lactated Ringer’s solution, and good old fashioned blood (especially when blood loss is responsible for the decreased volume!).
That’s great! But what do we do if we assess the patient’s volume status and come to the conclusion that it is sufficient, and the patient is STILL hemodynamically unstable? Not to fear… Let’s move on to the next tenet – systemic vascular resistance.
Systemic Vascular Resistance (SVR) and Afterload
The systemic vascular resistance is the resistance that blood sees as it travels through the arterial system. The vascular resistance varies depending on the etiology and time frame of the hemodynamic instability.
For example, patients in the initial stages of hypovolemic shock (ie: acute blood loss) the SVR will be elevated as the arteries constrict to maintain blood pressure; however, once the blood volume reaches critically low levels even constriction of the arteries is not enough to maintain the SVR. At this point the blood pressure will drop precipitously.
In contrast, patients in septic shock have a decreased systemic vascular resistance as the blood vessels dilate pathologically in response to infection. The result is the same as the above example: the blood pressure drops.
So how do we treat a decrease in systemic vascular resistance? Assuming intravascular volume (ie: preload) is adequate we sometimes need to "help" the patient constrict their blood vessels so they can maintain an adequate blood pressure. We do this using medications known as "pressors". The most commonly used pressors are norepinephrine (Levophed®), phenylephrine (Neosynephrine®), vasopressin, and dopamine.
Pressors should only be used once a patient has been treated with volume resuscitation. In other words, it does not make sense to help someone constrict their blood vessels when they have no intravascular volume to constrict around! This is why SVR is the 2nd (and not the 1st) tenet of managing someone with hemodynamic instability.
Heart Rate
Once intravascular volume status and vascular resistance have been dealt with, the next step is to monitor the heart rate. Patients who are excessively tachycardic (ie: high heart rate) or bradycardic (ie: low heart rate) can also be hemodynamically unstable. Let’s explain…
During tachycardia the left ventricle does not have as much time to fill with blood. Therefore, each beat of the heart ejects less blood than normal. If the tachycardia is severe enough this can cause hemodynamic instability and decreased blood flow. Likewise, in patients who are excessively bradycardic the amount of blood ejected over a specific unit of time (aka: cardiac output) may not be enough to sustain the bodies need for blood.
Patients who are excessively tachycardic can be treated with beta blockers assuming there is no other cause readily identified. It is also important to remember that tachycardia may be a sign of decreased intravascular volume! Therefore, the patient’s excessive heart rate may correct when the preload is corrected (again, always start at tenet number 1!).
Bradycardia can be treated with atropine. Atropine is a molecule that stimulates heart rate by blocking the actions of acetylcholine. It is also important to note that bradycardia is sometimes a "physiologic" response to the administration of pressors. For example, phenylephrine often causes a "reflex" bradycardia.
Heart Rhythm
Just like abnormal heart rates, abnormal heart rhythms can also cause hemodynamic instability. The most commonly encountered abnormal rhythms in hemodynamically unstable patients are atrial fibrillation, atrial flutter, heart block, ventricular fibrillation, and ventricular tachycardia.
Abnormal heart rhythms contribute to hemodynamic instability by decreasing the filling capacity of the heart. For example a patient in rapid atrial fibrillation will not have enough time between heart beats to load their left ventricle with blood.
The treatment of the various cardiac arrhythmias depends on the type of rhythm. It is important to note that abnormal rhythms may not have a significant impact on hemodynamic instability, and may need no additional treatment. This is most commonly encountered in people who are in atrial fibrillation, but their rate is well controlled.
Contractility
You’ve fixed intravascular volume, corrected the systemic vascular resistance, examined the heart rate and rhythm and you still can not stabilize the patient. Under these circumstances you have to think about the heart itself. It may not be ejecting blood efficiently or effectively enough to ensure cardiovascular stability.
Under these circumstances you need to order an echocardiogram to assess the heart’s function. If the heart is not holding its own it may need a little help. Medications such as epinephrine, dopamine, dobutamine, milrinone, and digoxin can help give the heart a little extra “squeezing power” to improve cardiac output.
Overview
The management of hemodynamic instability always begins with an assessment of intravascular volume (preload). If repleting intravascular volume does not fix the problem then the next step is to look at systemic vascular resistance. Does the patient need a little help constricting those blood vessels? If not, than you have to move quickly to assessing heart rate and rhythm. Finally, when all else fails, we have to assess the contractility of the heart. Maybe it isn’t pumping as hard or as efficient as it should be.
Correcting hemodynamic instability in the above order is both a quick and efficient framework for thinking about the unstable patient. Often times the physician will drop quickly down the list as they recognize a problem immediately (ie: rapid atrial fibrillation) and this is ok!!! The important thing is to have a framework for thinking about cardiovascular instability.
References and Resources
- Stiell IG, Macle L. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: management of recent-onset atrial fibrillation and flutter in the emergency department. CCS Atrial Fibrillation Guidelines Committee. Can J Cardiol. 2011 Jan-Feb;27(1):38-46.
- Bozza FA, Carnevale R, Japiassú AM, et al. Early fluid resuscitation in sepsis: evidence and perspectives. Shock. 2010 Sep;34 Suppl 1:40-3. Review.
- Price S, Uddin S, Quinn T. Echocardiography in cardiac arrest. Curr Opin Crit Care. 2010 Jun;16(3):211-5.
- Naeem N, Montenegro H. Beyond the intensive care unit: a review of interventions aimed at anticipating and preventing in-hospital cardiopulmonary arrest. Resuscitation. 2005 Oct;67(1):13-23.
- Sevransky J. Clinical assessment of hemodynamically unstable patients. Curr Opin Crit Care. 2009 Jun;15(3):234-8.