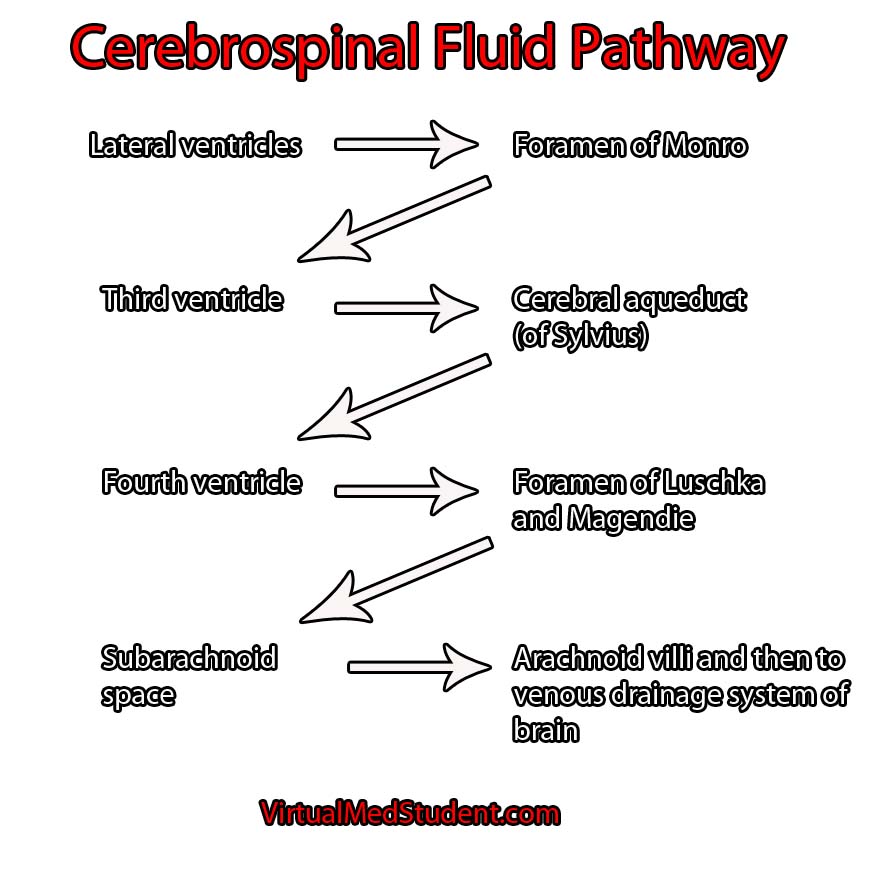
The vein of Galen is located deep within the brain. The internal cerebral veins, basal veins of Rosenthal, atrial veins, and precentral cerebellar veins join together to form the vein of Galen.
As expected, the vein of Galen drains blood from deeply located brain structures. The vein of Galen then connects with the inferior sagittal sinus to form the straight sinus; blood then drains from the straight sinus into the transverse and sigmoid sinuses, where it eventually finds its way into the internal jugular veins and back to the heart.
Interestingly, a "vein of Galen" malformation is not actually a malformation of the true vein of Galen; the term is a misnomer. It is actually a malformation of primitive fetal anatomical structures that normally regress during development. These primitive structures include a dilated venous structure, as well as "feeding" arteries. Therefore, vein of Galen malformations represent true arteriovenous fistulas; in other words, blood moves directly from an artery to a vein without an intermediary capillary bed.
Between the 3rd and 11th weeks of fetal development a large primitive vein known as the median prosencephalic vein of Markowitz drains the deepest parts of the brain. As the brain develops, the internal cerebral veins annex the territory normally drained by the anterior portion of this vein. As a result, this portion of the median prosencephalic vein regresses. The internal cerebral veins then plug in to the posterior portion of the median prosencephalic vein, which becomes the "true", or "normal", vein of Galen.
The most common arterial "feeders" of the malformation can also be explained by aberrant embryology. During early fetal brain development the distal branches of the anterior cerebral arteries (ie: the pericallosal branches) make connections with the posterior cerebral arteries. These connections usually regress to form the anterior and posterior circulations, which are connected to one another via the posterior communicating arteries.
So how does a vein of Galen malformation form? In some infants the median prosencephalic vein of Markowitz does not regress like it should. As a result, a large abnormal venous midline pouch remains. It also retains its primitive arterial blood supply from the distal branches of the anterior cerebral artery (ie: pericallosal branches), anterior choroidal arteries, posterior communicating arteries, and branches of the posterior cerebral arteries (ie: posterior choroidal arteries).
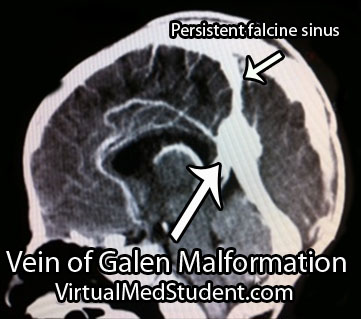
Vein of Galen malformations are also associated with other abnormalities in the venous structure of the brain. Not uncommonly, the straight sinus is absent or severely narrowed. As a result venous blood drains into a persistent falcine sinus, which is a structure that normally regresses in-utero.
To summarize, vein of Galen malformations are primitive direct arteriovenous fistulas. They are composed of a dilated venous pouch (ie: the median prosencephalic vein of Markowitz) with any combination of anterior and posterior circulation feeding arteries.
Signs and Symptoms
Signs and symptoms depend on the severity of the malformation. Severe malformations present in new borns with high output cardiac failure. This is because so much blood is being shunted into the malformation that the heart cannot keep pace!
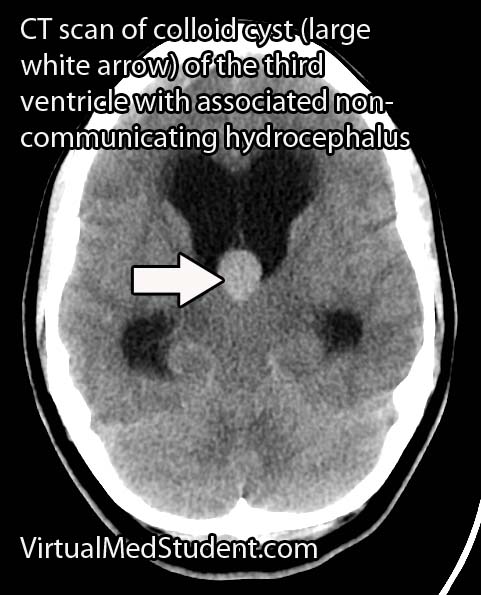
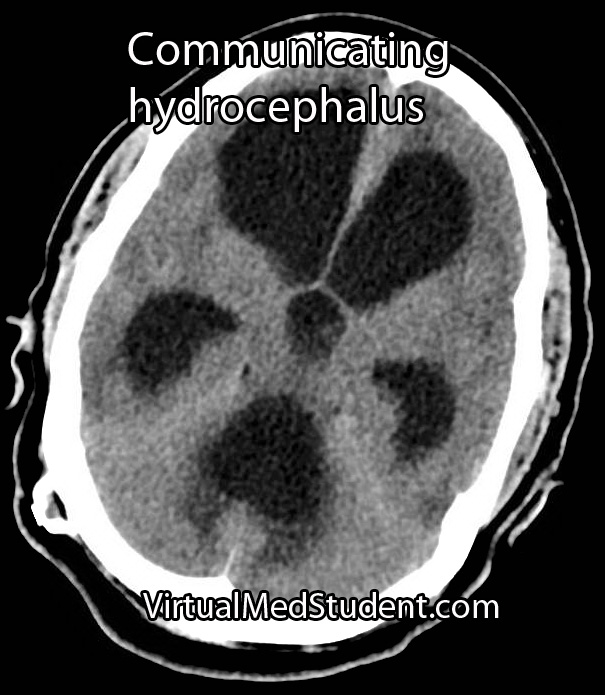
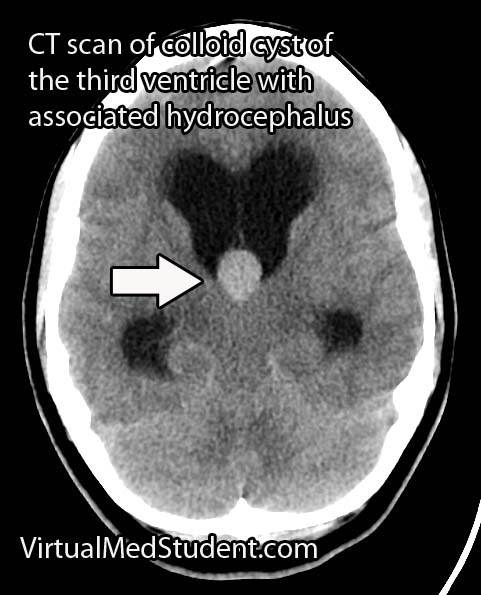
Less significant malformations present later in infancy with a rapidly enlarging head circumference secondary to hydrocephalus, developmental delay, and seizures. The increase in venous blood pressure within the head can cause a "melting brain" syndrome in which the white matter of the brain fails to develop properly. This can lead to severe mental retardation later in life if left untreated.
Diagnosis
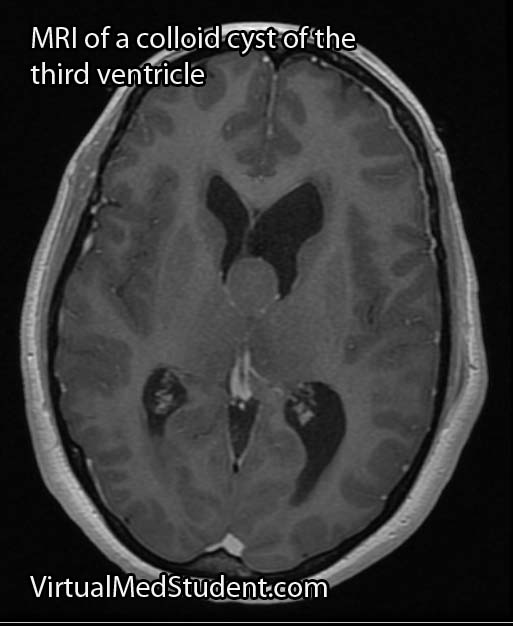
Diagnosis is made with a combination of MRI, CT, and diagnostic angiograms. MR venograms can show the dilated venous pouch, as well as associated venous anomalies.
CT angiograms can show associated arterial feeding vessels. Formal diagnostic angiograms are the gold standard test; they delineate both the spatial and temporal relationship of the arterial feeding vessels to the venous pouch.
Formal catheter angiograms are also necessary to distinguish true vein of Galen malformations from arteriovenous malformations of the adjacent brain tissue.
Treatment
Treatment is dependent on the age of the child as well as the severity of the malformation. The most commonly used grading system developed by Dr. Lasjaunias is known as the Bicetre score. It takes into account the child’s cardiac, pulmonary, hepatic (liver), and renal (kidney) function. Lower scores indicate more severe disease with poorer outcomes.
The Bicetre score also dictates the optimal time for treatment. If the score is very low then aggressive treatment, even in the neo-natal period, may be indicated to try and prevent death and severe disability. Higher scores are typically treated later in life; however, worse outcomes, in terms of mental retardation, have been illustrated if treatment is delayed.
Most of these lesions are treated endovascularly (ie: from inside the blood vessels). The arterial feeders are embolized with a glue like material, which ultimately shuts down the fistula in an attempt to restore normal venous pressures. The vein itself may also be filled with tiny metal coils to help reduce flow through the fistula; this is known as trans-venous endovascular therapy.
Surgical ligation of the arterial feeders has mostly become a treatment of the past. Radiation therapy with Gamma Knife has also been used in some cases; it is showing some promise as an alternative treatment modality in select cases.
Overview
Vein of Galen malformations are fetal abnormalities in the brain’s normal venous drainage. They represent true arteriovenous fistulas. They are composed of a dilated median prosencephalic vein of Markowitz and numerous arterial feeding vessels. Feeders may come from the anterior cerebral arteries, posterior cerebral arteries, or posterior communicating arteries. Symptoms are usually from high output heart failure in the neonatal period; older infants and children suffer from increasing head circumference, seizures, and developmental delay. Treatment is usually with endovascular techniques.
References and Resources
- Mortazavi MM, Griessenauer CJ, Foreman P, et al. Vein of Galen aneurysmal malformations: critical analysis of the literature with proposal of a new classification system. J Neurosurg Pediatr. 2013 Sep;12(3):293-306.
- Pearl M, Gomez J, Gregg L, Gailloud P. Endovascular management of vein of Galen aneurysmal malformations. Influence of the normal venous drainage on the choice of a treatment strategy. Childs Nerv Syst. 2010 Oct;26(10):1367-79.
- Geibprasert S, Krings T, Armstrong D, et al. Predicting factors for the follow-up outcome and management decisions in vein of Galen aneurysmal malformations. Childs Nerv Syst. 2010 Jan;26(1):35-46.
- Heuer GG, Gabel B, Beslow LA, et al. Diagnosis and treatment of vein of Galen aneurysmal malformations. Childs Nerv Syst. 2010 Jul;26(7):879-87.
. Sixth Edition. New York: Thieme, 2006. Chapter 25.