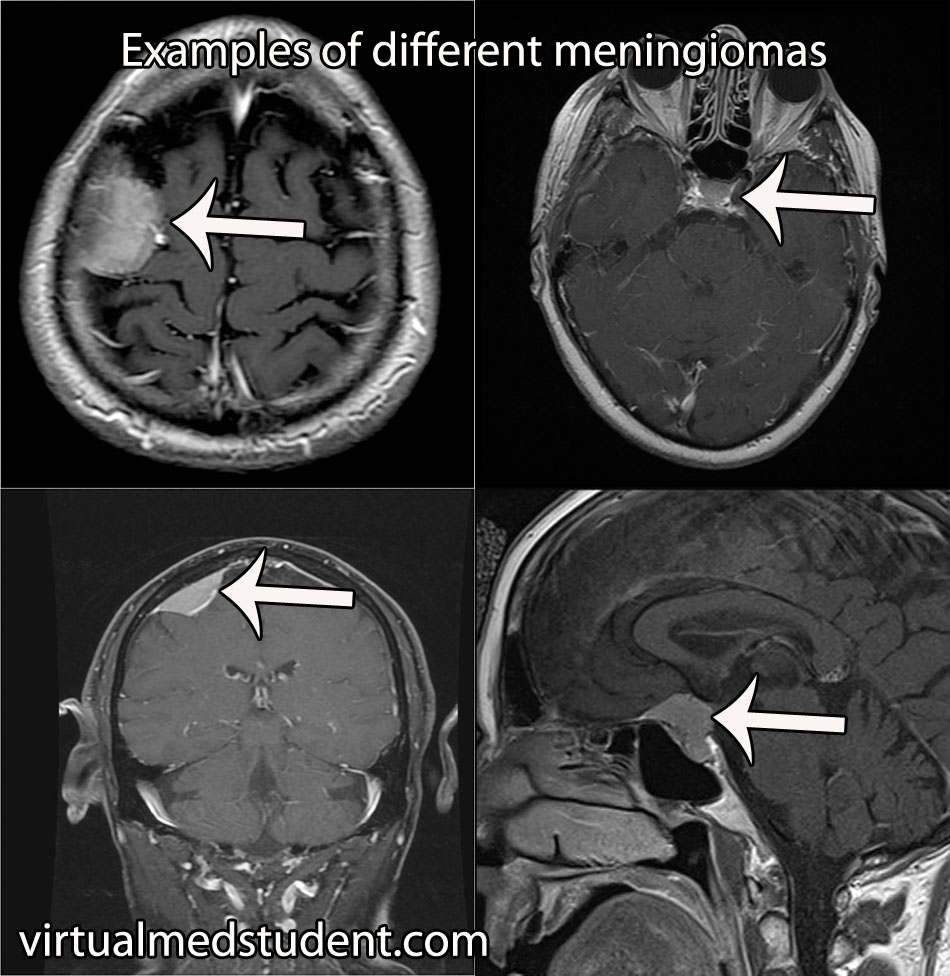
Each A1 segment branches into an A2 segment and into the anterior communicating artery. The anterior communicating artery connects each anterior cerebral arteries’ A1 segment together to form a circle (see schematic below).
The anterior communicating artery is one of the most common sites for intracranial aneurysm formation. Patients at risk for developing aneurysms include those with atherosclerosis, those with a family history of intracranial aneurysms, those with a history of hypertension or collagen vascular disease, and those with polycystic kidney disease. Smokers are also at a higher risk of developing aneurysms.
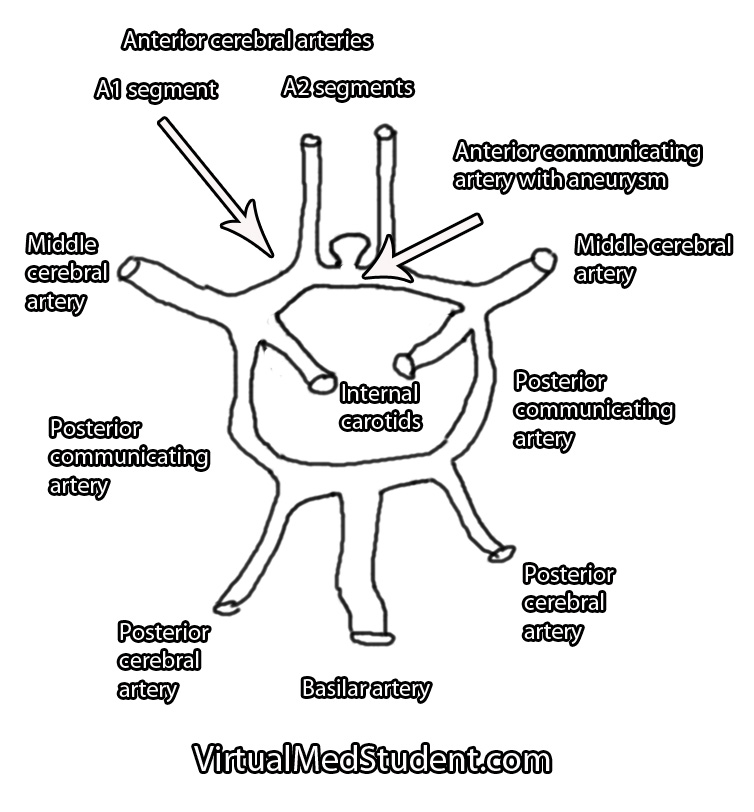
This thinning allows turbulent blood flow to form out-pouchings in the vessel wall. Typically these out-pouchings occur at points where blood vessels branch.
Signs and Symptoms
Anterior communicating artery aneurysms commonly present after a subarachnoid hemorrhage, which can cause a variety of signs and symptoms. The most common being a severe headache, although cranial nerve dysfunction, stroke, coma, and death can also occur.
Less commonly, aneurysms in this location can compress the optic chiasm or optic nerves leading to problems with vision.
How Do You Diagnose Aneurysms
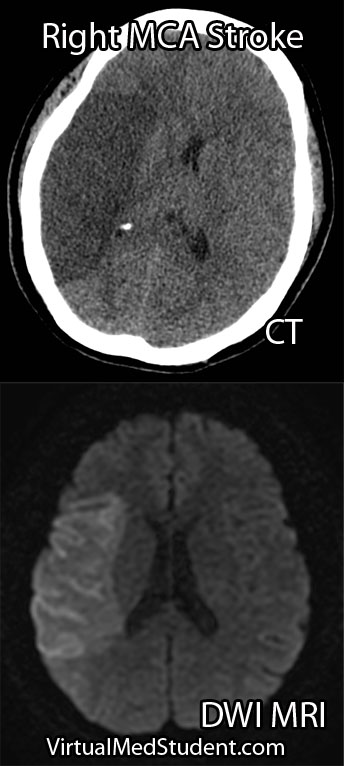
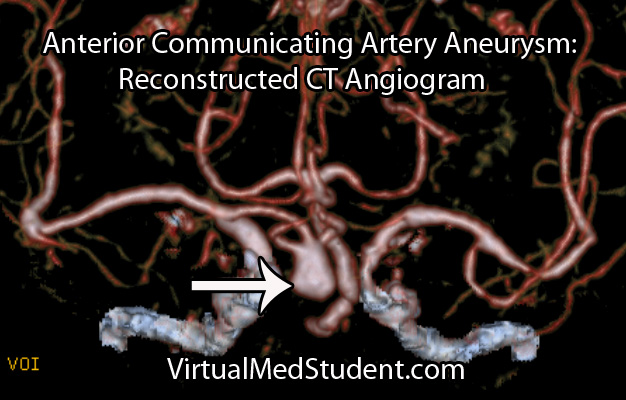
Anterior cerebral artery aneurysms are most commonly diagnosed after a subarachnoid hemorrhage when a patient presents with the "worst headache of their life". The best imaging methods for diagnosing these aneurysms are CT angiograms (see image below), MR angiograms, and formal cerebral angiograms.
Treatment
Like other intracranial aneurysms, anterior communicating artery aneurysms may be clipped or coiled. Clipping of an aneurysm involves an open surgical procedure where the surgeon dissects down to the aneurysm and places a clip across its neck. This excludes it from the circulation and prevents it from rupturing.
Aneurysms may also be treated from inside the blood vessel. In this procedure a catheter is threaded from the femoral artery in the groin up towards the location of the aneurysm. Small metallic coils are placed within the dome of the aneurysm, which also excludes it from the normal circulation.
Regardless of how the aneurysm is treated – either with clipping or coiling – the end result is that the aneurysm is excluded from the normal circulation. This prevents it from rupturing.
The merits of clipping versus coiling are still under debate. Ultimately, the treatment depends on the size and location of the aneurysm, as well as other medical problems that the patient may have.
The Highlights…
Anterior communicating artery aneurysms are the most common intracranial aneurysm. They typically present after rupturing into the subarachnoid space and/or adjacent frontal lobes. They are diagnosed using CT angiograms or formal cerebral angiography. Treatment is with clipping and/or coiling.
Related Readings
- Subarachnoid Hemorrhage: Aneurysms, Vasospasm, and Hyponatremia Brain Boo Boos: Cerebrovascular Accidents (Stroke)
- Hypertension: Understanding and Managing High Blood Pressure
- Atherosclerosis: Gruel and Hardening
Not Satisfied? More Reading on the Subject…
- Bederson JB, Awad IA, Wiebers DO, et al. Recommendations for the management of patients with unruptured intracranial aneurysms. Stroke 2000;31:2742-2750. li>Hunt WE, Hess RM. Surgical Risk as Related to Time of Intervention in the Repair of Intracranial Aneurysms. Journal of Neurosurgery 1968; 28:14-20.
- Brisman JL, Song JK, Newell DW. Cerebral Aneurysms. NEJM 2006; 355:928-939.
- Kumar V, Abbas AK, Fausto N. Robbins and Cotran Pathologic Basis of Disease. Seventh Edition. Philadelphia: Elsevier Saunders, 2004.
- Frontera JA. Decision Making in Neurocritical Care. First Edition. New York: Thieme, 2009.
- Greenberg MS. Handbook of Neurosurgery. Sixth Edition. New York: Thieme, 2006. Chapter 25.