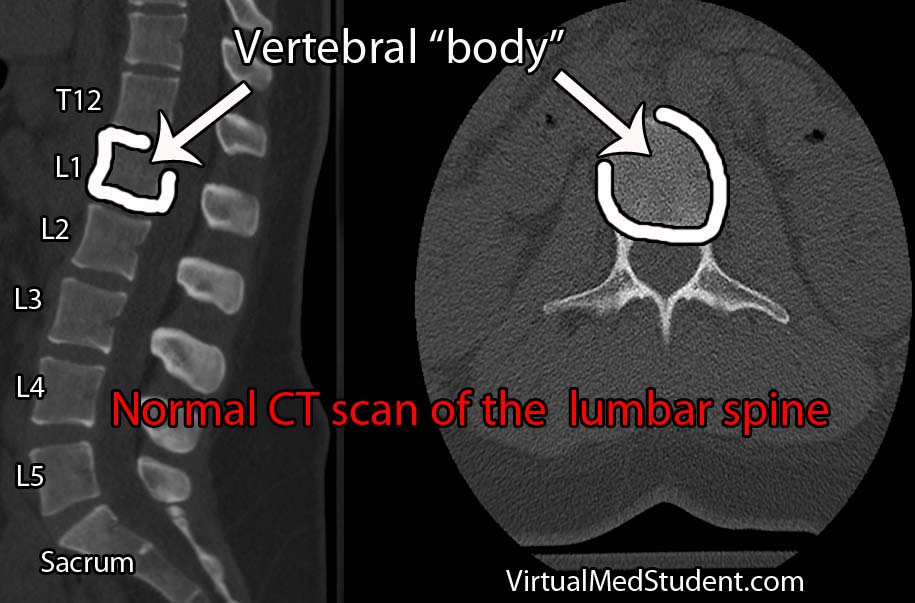
The cauda equina is an anatomical term given to all of the nerves that dangle off the end of the spinal cord. In Latin, the term cauda equina means "horse’s tail". The dangling nerves are housed in the thecal sac, and are normally bathed in a sea of cerebral spinal fluid. Each of the nerves in the cauda equina eventually branches from the thecal sac. From there they exit the spinal column and travel to their respective target (ie: muscle, skin, organ, etc.).
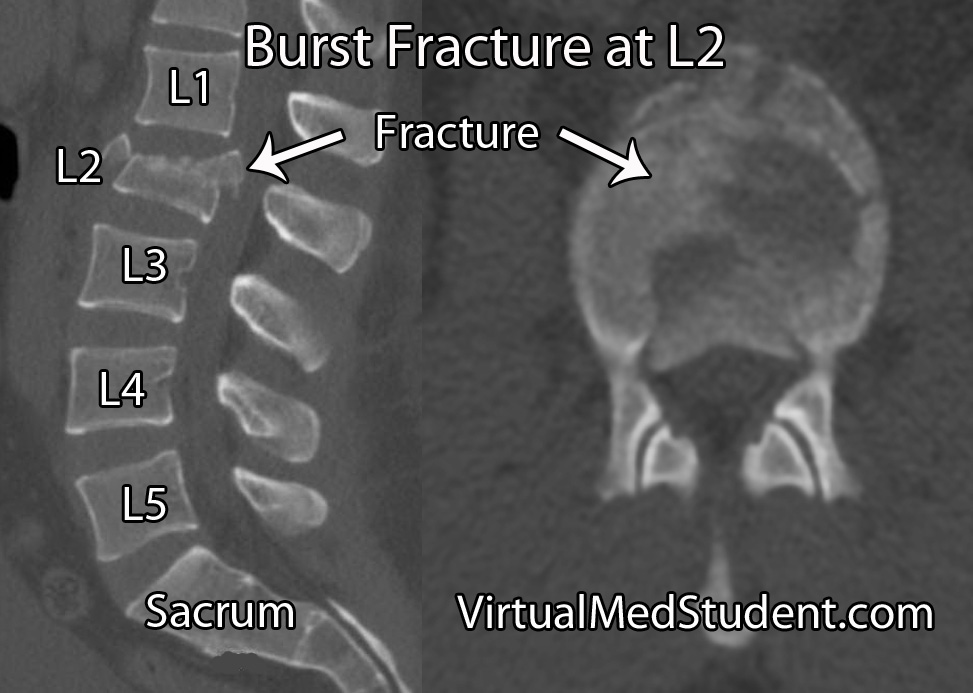
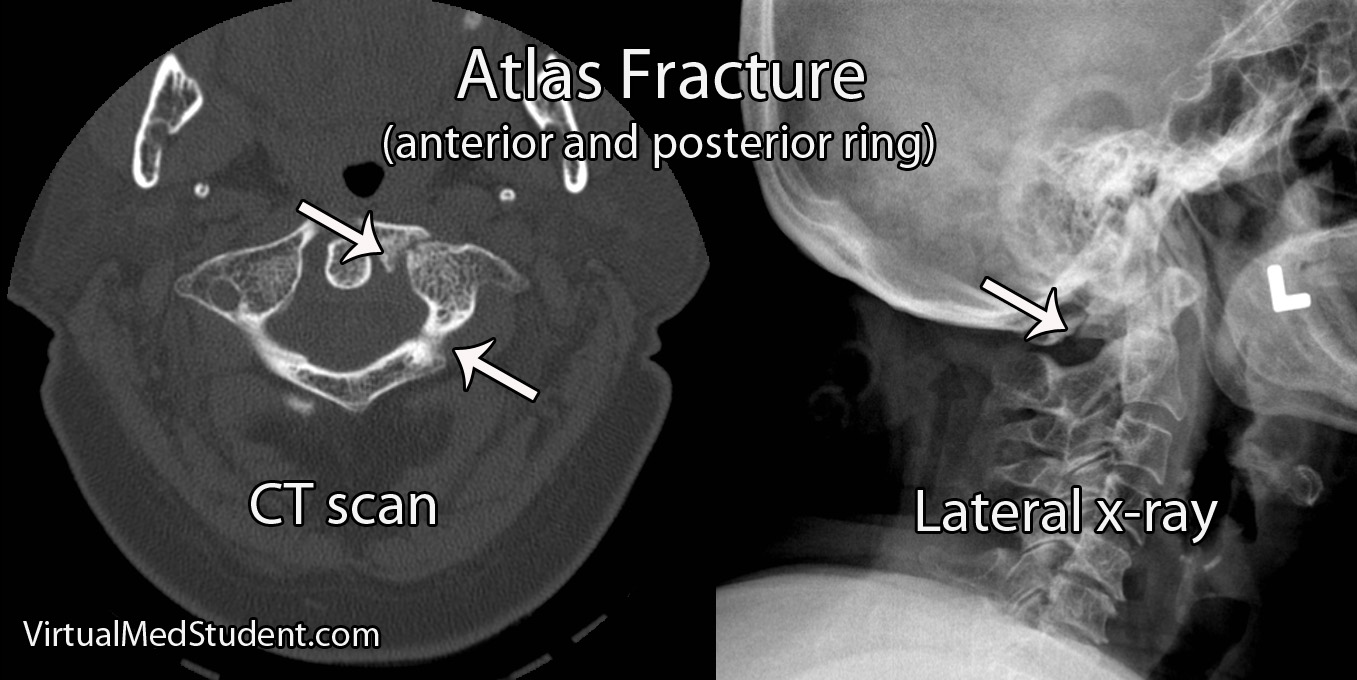
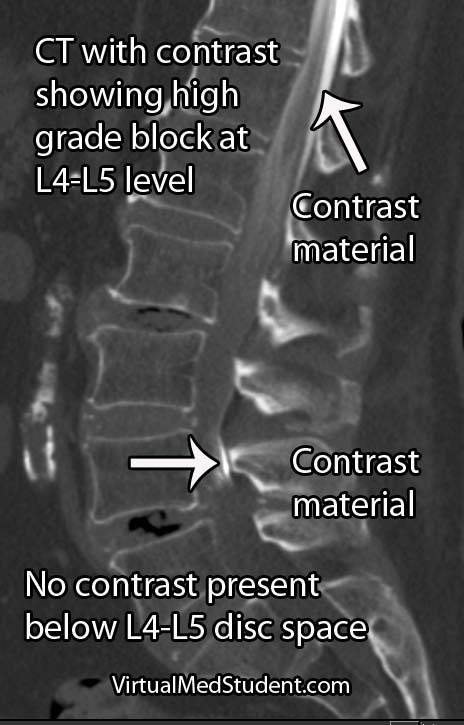
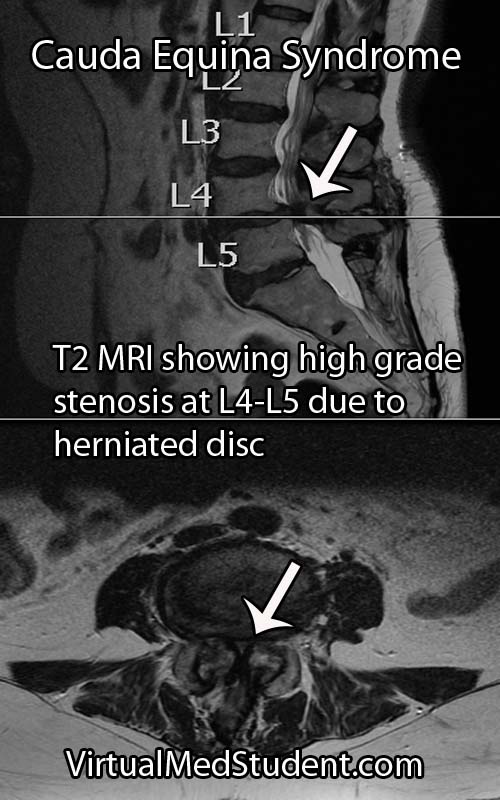
Cauda equina syndrome occurs when the nerves in the "horse’s tail" get squished by some pathologic process. The most common pathology is a large herniated intervertebral disc that pushes back on the thecal sac. Other causes include tumors, blood clots (ie: hematomas), traumatic injuries such as burst fractures or fracture dislocations, severe lumbar stenosis, as well as abscesses.
Regardless of the cause, when the nerves get impinged they are unable to perform their functions. This leads to the classic signs and symptoms of cauda equina discussed below.
Signs and Symptoms
The classic teaching is that cauda equina syndrome presents with the acute onset of lower extremity weakness, poop incontinence (ie: fecal incontinence if you want to be "scientific" about it), urinary retention (ie: patient cannot pee), loss of leg reflexes, low back pain, sexual dysfunction, and loss of sensation, especially in the "saddle" and peri-anal region. Please note urinary RETENTION is a key factor and one that is often mixed up.
It is important to note that patients with cauda equina syndrome rarely present with all of these symptoms. The most worrisome symptoms are sudden weakness and bowel or bladder issues.
Additionally, radicular type symptoms such as numbness and tingling, or sharp pains down the legs can also be present.
Classic Signs and Symptoms
of Cauda Equina Syndrome:
– Lower extremity weakness
– Bowel incontinence
– Urinary retention
– Loss of reflexes
– Saddle anesthesia
– Sexual dysfunction
It is also important to distinguish between acute and chronic forms of the syndrome. Acute symptoms occur with rapid onset and require emergent evaluation. However, many patients have evidence of chronic dysfunction of the nerves that compose the cauda equina; their symptoms have been slowly evolving over months or years (ie: think older people with lumbar stenosis).
The time frame in which cauda equina symptoms develop is important for determining optimal treatment, and providing patients with a realistic prognosis for recovery.
Diagnosis
In addition to a thorough neurological examination, patients with suspected cauda equina syndrome should also have a post void residual (PVR) measurement. PVRs help diagnose urinary retention, which is a worrisome finding if present.
Treatment
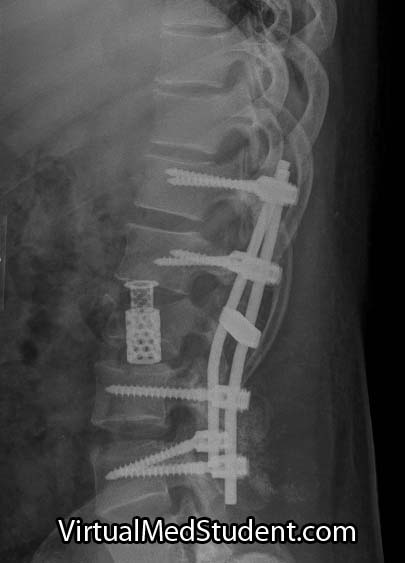
"True" acute cauda equina syndrome caused by a large mass pushing on the nerve roots is managed with urgent surgical decompression. Typically, a laminectomy (ie: a procedure in which part of the bone in the back of the spinal column is removed) is performed to relieve pressure on the thecal sac.
Other therapies including antibiotics for infections, chemotherapy for tumors, and steroids for inflammatory causes are also used.
Overview
Cauda equina syndrome is caused by compression of the nerves in the thecal sac. Classic symptoms include weakness, saddle anesthesia, fecal incontinence, and urinary retention. Furthermore, both acute and chronic forms of cauda equina syndrome exist based on the rapidity of symptom onset. Diagnosis is based on clinical signs and symptoms in conjunction with MRI or CT myelogram imaging showing compression of the nerves.
References and Resources
- Korse NS, Jacobs WC, Elzevier HW, et al. Complaints of micturition, defecation and sexual function in cauda equina syndrome due to lumbar disk herniation: a systematic review. Eur Spine J. 2012 Dec 13.
- Fraser S, Roberts L, Murphy E. Cauda equina syndrome: a literature review of its definition and clinical presentation. Arch Phys Med Rehabil. 2009 Nov;90(11):1964-8.
- Mauffrey C, Randhawa K, Lewis C, et al. Cauda equina syndrome: an anatomically driven review. Br J Hosp Med (Lond). 2008 Jun;69(6):344-7.
- Shi J, Jia L, Yuan W, et al. Clinical classification of cauda equina syndrome for proper treatment. Acta Orthop. 2010 Jun;81(3):391-5.
- Gitelman A, Hishmeh S, Morelli BN, et al. Cauda equina syndrome: a comprehensive review. Am J Orthop (Belle Mead NJ). 2008 Nov;37(11):556-62.
- Baehr M, Frotscher M. Duus’ Topical Diagnosis in Neurology: Anatomy, Physiology, Signs, Symptoms. Fourth Edition. Stuttgart: Thieme, 2005.