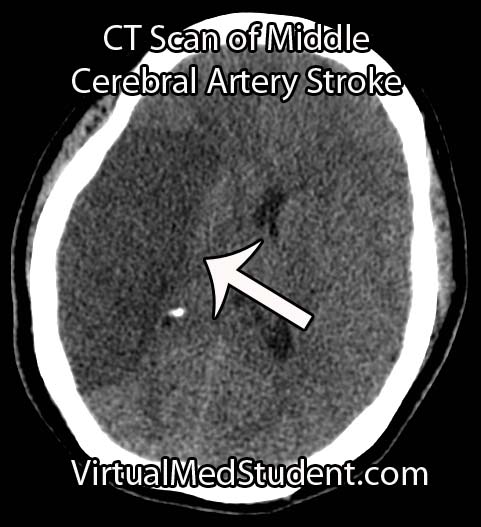
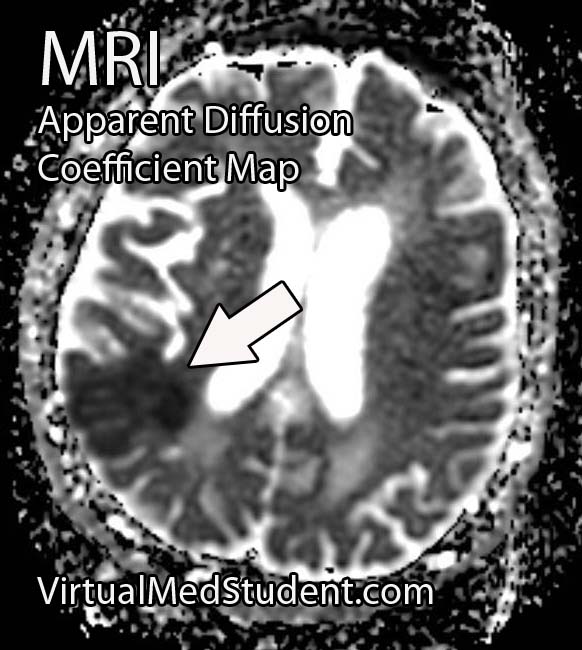
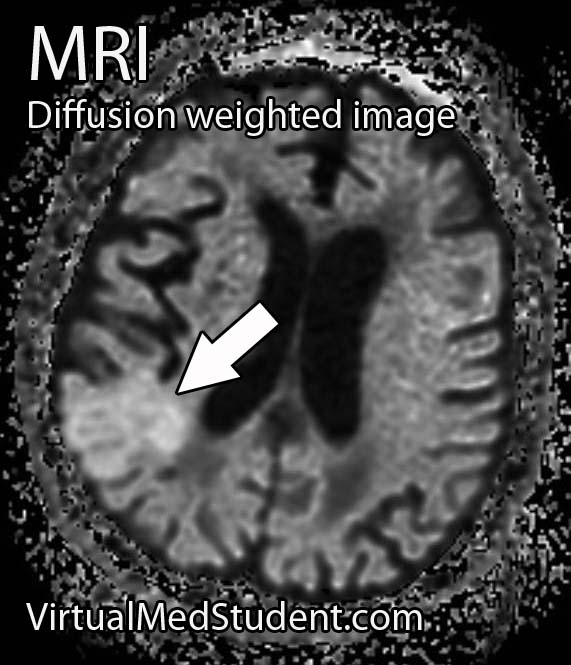
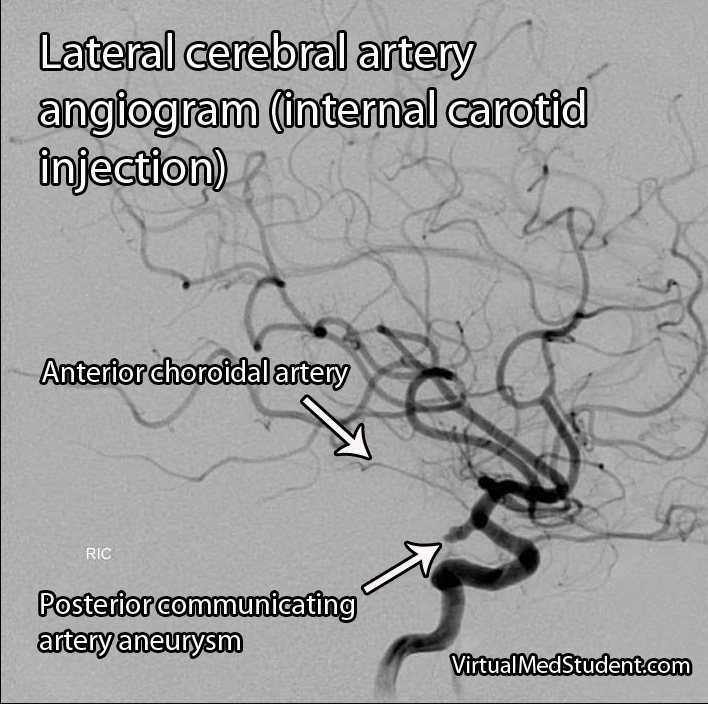
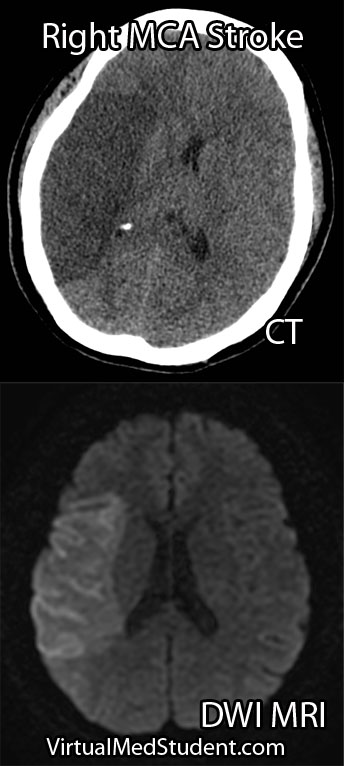
Middle cerebral artery (MCA) strokes occur when the MCA or its branches are occluded. With occlusion, blood, and along with it, oxygen and nutrients fail to reach the brain. If blood flow is not restored quickly the affected brain tissue dies leading to permanent neurological injury.
Risk factors for MCA strokes are the same for other strokes. They include hypertension, diabetes, smoking, atrial fibrillation, and a whole slew of hypercoagulable states (ie: pathologic increases in the bodies’ propensity to form blood clots).
In order to understand MCA strokes we have to first appreciate the anatomy of the MCAs, as well as the brain that they serve. The MCAs are subdivided into four parts. The M1 segment is one of the terminal branches of the internal carotid artery. The MCAs then become progressively more narrow and form more and more branches as they reach out towards the cortical surfaces of the brain. The most distal portions of the MCA are deemed the M4 branches. The lateral lenticulostriate arteries are small branches that branch from the M1 segments; these small arteries feed some of the deeper structures of the brain.
Importantly, the MCAs and their branches provide blood flow to an extremely large portion of the brain. These areas include the lateral and inferior frontal lobes, superior portion of the temporal lobes, insula, and lateral parietal lobes. Branches of the first portion of the MCAs (aka: the lateral lenticulostriate arteries) also provide blood flow to the deep sections of the brain including the putamen, head and body of the caudate, external globus pallidus, and parts of the posterior limb of the internal capsule.
The segmental anatomy of the MCAs is important because strokes that occur in the outer segments (ie: M3 and M4) cause less neurological injury than the inner MCA segments (ie: M1 and M2). This is because less total brain volume is affected by outer segment strokes.
The most common cause of MCA strokes are clots that break off and travel from the heart or the carotid arteries to the MCA (aka: emboli). Less commonly, a blood clot will form directly in the MCA itself (aka: thrombus). Atherosclerotic disease is the most common cause of thrombus formation in the MCA and atrial fibrillation (an abnormal heart rhythm) is the most common cause of emboli from the heart.
Signs and Symptoms
Middle cerebral artery strokes present with one of two types of syndromes depending on which MCA – right or left – is involved, as well as which segments of the MCA are involved.
In the worst case scenario, a stroke of the right or left M1 segment of the MCA causes weakness of the opposite side of the body. This is a result of cortical damage to the primary motor cortex as well as possible infarction of the posterior limb of the internal capsule (ie: the location of descending motor tracts). MCA strokes usually present with face and arm weakness that is worse than leg weakness. Remember that the motor cortex that controls leg function is served by the anterior cerebral arteries.
M1 strokes also cause decreased ability to feel sensation on the opposite side of the body as a result of damage to the parietal lobes.
Damage to the optic radiations, which course in the parietal (Baum’s loop) and temporal lobes (Meyer’s loop) can cause problems with vision. Finally, injuries to the frontal eye fields cause the eyes to deviate towards the side of the stroke (ie: the frontal eye fields normally allow you to make fast eye movements in the opposite direction, therefore damage prevents patients from looking to the non-affected side).
Right and left sided M1 strokes will give you all of the above symptoms; however, laterality can be determined based on specific symptoms caused by only a left or a right sided strokes.
The poor patients with left M1 strokes have a decreased ability to speak and/or understand language because of damage to Broca’s area in the left frontal lobe (speech production) and Wernicke’s area in the left temporal lobe (speech comprehension). Remember this is in addition to all the other stuff above.
Right M1 strokes can cause "anosognosia", in which the patient is unaware of certain deficits they may have; these patients also often fail to recognize the left side of their body (ie: they "neglect" or fail to appreciate the entire left side of the world) and may have difficulty appreciating people or objects presented in their left visual field.
Diagnosis
Diagnosis of MCA strokes are based on symptoms, CT scans, and MRI images. CT and MR angiograms frequently show the blocked blood vessel causing the stroke. CT perfusion scans are a newer technology that give information regarding the amount of blood flow to affected brain tissue.
Treatment
Treatment depends on the timing of the stroke. If the patient presents within 3 hours of symptom onset, and the head CT reveals no bleeding, than intravenous tissue plasminogen activator (tPA) may be given to help "break" up the blood clot causing the stroke.
Other treatments using catheter based approaches are frequently used in patients who are unable to receive tPA. Such treatments include mechanical clot removal with special catheter and wire devices. In addition, in patients more than 3 hours, but less than 6 hours out from symptom onset intra-arterial (not to be confused with intravenous) tPA may be used.
Less commonly, large "malignant" MCA strokes may cause significant swelling, which can put pressure on the brainstem. These patients sometimes undergo an open surgical procedure known as a "craniectomy", in which the bone overlying the affected brain tissue is removed. This surgery allows the edematous brain tissue to swell outwards preventing it from herniating downwards towards vital brainstem structures.
Overview
Middle cerebral artery strokes are most commonly caused by blood clots that break off from the heart or carotid artery. Symptoms of MCA strokes depend on the segment involved, as well as which MCA (right versus left) is involved. Diagnosis is made with a combination of MRI, CT, and symptomatology. Treatment consists of intravenous or intra-arterial tPA and/or mechanical clot removal depending on the time frame of the symptoms.
Related Articles
- Atherosclerosis: Gruel and Hardening
- Internal Capsule: Some Pricey Brain Real Estate
- The Direct Basal Ganglia Pathway
- Subarachnoid Hemorrhage: Aneurysms, Vasospasm, and Hyponatremia
References and Resources
- Taussky P, Tawk RG, Daugherty WP, Hanel RA. Medical therapy for ischemic stroke: review of intravenous and intra-arterial treatment options. World Neurosurg. 2011 Dec;76(6 Suppl):S9-15. Review.
- Tenser MS, Amar AP, Mack WJ. Mechanical thrombectomy for acute ischemic stroke using the MERCI retriever and penumbra aspiration systems. World Neurosurg. 2011 Dec;76(6 Suppl):S16-23.
- Baehr M, Frotscher M. Duus’ Topical Diagnosis in Neurology: Anatomy, Physiology, Signs, Symptoms
. Fourth Edition. Stuttgart: Thieme, 2005.
- Ropper A, Samuels M. Adams and Victor’s Principles of Neurology, Ninth Edition
. McGraw-Hill Professional; 9 Edition (March, 2009).