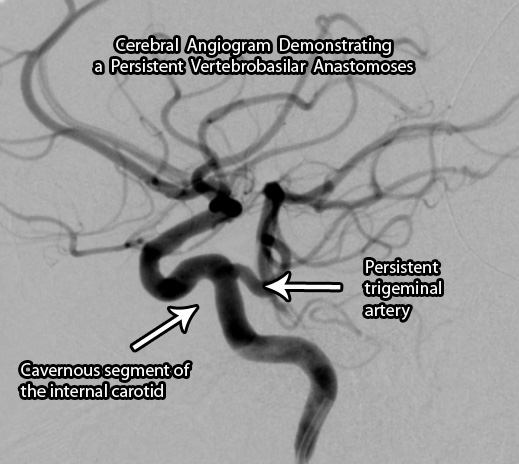
The post-natal blood vessels of the brain are broadly broken up into the anterior (carotid) and posterior (vertebro-basilar) circulations. After birth, these circulations are, at least in theory, connected together via a circle of blood vessels at the base of the brain known as the Circle of Willis. The vessels that complete this circle are the paired posterior communicating arteries.
Prior to the development of the posterior communicating arteries in-utero, there are a series of blood vessels that appear and disappear as the arterial tree of the brain develops into its post-natal form. These vessels form transient connections between the anterior (carotid) and posterior (vertebro-basilar) circulations.
In some individuals these transient blood vessels do not regress and remain patent. These embryonic connections are collectively known as the "persistent carotid-vertebrobasilar anastomoses".
There are four known anastomoses. They are the persistent trigeminal artery, otic (acoustic) artery, hypoglossal artery, and proatlantal intersegmental artery. Some authors classify fetal posterior communicating arteries as a fifth anastomoses, but given how common these are we have dedicated an entire article to this variant. We will only discuss the remaining four.
The Anastomoses
The most common of the persistent anastomoses is the trigeminal artery. This artery connects the cavernous segment of the internal carotid artery directly to one of the vertebral arteries. Saltzman further categorized these anastomoses into two types. A type I vessel has an absent ipsilateral (ie: same side) posterior communicating artery; whereas a type II has an ipsilateral fetal posterior communicating artery present. Individuals with trigeminal arteries are also at increased risk of developing aneurysms throughout their cerebral vasculature.
The least commonly seen anastomoses is the otic (acoustic) artery. This vessel arises from the petrous internal carotid artery and plugs into the basilar artery. This is the first vessel to regress in-utero and is rare to see in post-natal life.
The proatlantal intersegmental artery is a link between the cervical carotid artery and the vertebral artery. Like the trigeminal artery, it is further sub-divided into two different types. In a type I intersegmental artery the vessel arises from the internal carotid artery; whereas in a type II intersegemental artery the vessel arises from the external carotid artery.
The hypoglossal artery arises from the cervical internal carotid artery and plugs in to the basilar artery.
| Persistent Carotid Vertebrobasilar Anastomoses | |
| Name | Vessels Connected |
| Trigeminal artery | Cavernous internal carotid to vertebral artery |
| Otic (acoustic) artery | Petrous internal carotid to basilar artery |
| Proatlantal intersegmental artery | Type I – cervical internal carotid artery to vertebral artery Type II – external carotid artery to vertebral artery |
| Hypoglossal artery | Cervical internal carotid artery to basilar artery |
Overview
The persistent vertebrobasilar anastomoses are connections between the anterior and posterior cerebral circulations that fail to regress in-utero. Certain types are associated with increased risk of aneurysm formation. They are normally clinically silent and found incidentally during the work-up for other symptoms.
Related Articles
References and Resources
- Luh GY, Dean BL, Tomsick TA, Wallace RC. The persistent fetal carotid-vertebrobasilar anastomoses. AJR 172:1427-1432, 1999.
- Nolte J. The Human Brain: An Introduction to its Functional Anatomy
. Sixth Edition. Philadelphia: Mosby, 2008.
- Greenberg MS. Handbook of Neurosurgery
. Sixth Edition. New York: Thieme, 2006.
- Osborn AG. Introduction to Cerebral Angiography
. Second Edition. Lippincott Williams and Wilkins, 1980.