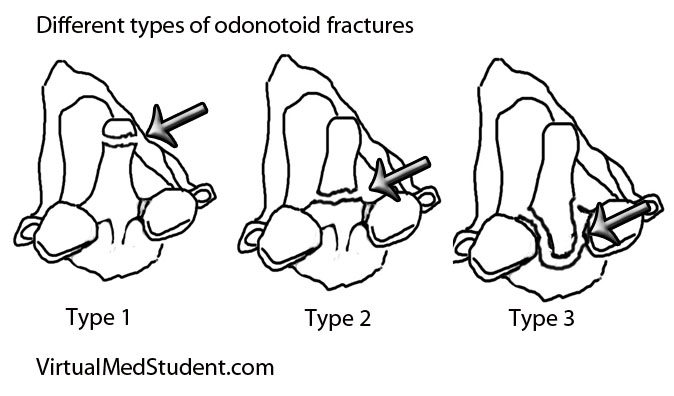
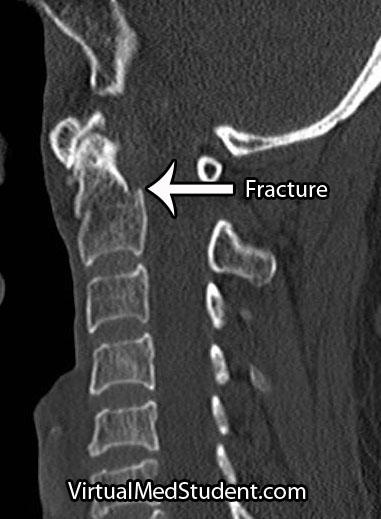
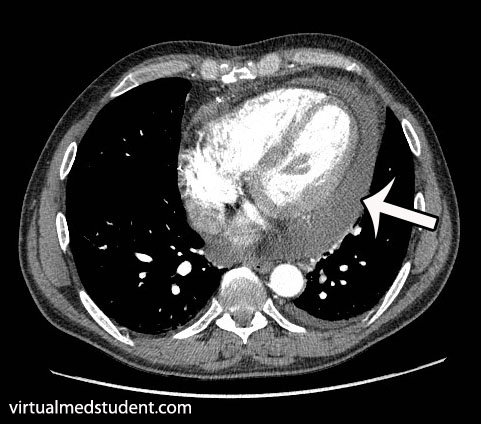
Lymphoma is a cancer that develops from cells in the body known as lymphocytes. Lymphocytes are a subcategory of white blood cells, which are the cells that help ward off infection. There are two different types of lymphocytes: B-cells and T-cells. The majority of lymphomas, including Hodgkin’s disease, stem from B-cells.
In Hodgkin’s disease a B-cell, for unknown reasons, becomes cancerous. The cell then makes many clones of itself. These cells bundle together to form a solid tumor known as a lymphoma.
Why B-cells in Hodgkin’s lymphoma become cancerous is not entirely known. One belief is that infection with Epstein-Barr virus (the same virus that causes infectious mononucleosis) can cause the cells to turn cancerous in genetically susceptible people. Other theories are that certain genetic translocations may be the underlying factor. As of yet, no particular theory has significant supporting data to call it the true cause. It is likely that the development of Hodgkin’s disease is multi-factorial.
Types
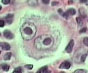
There are different types of Hodgkin’s lymphoma. They are based on unique histopathological (ie: what it looks like under a microscope) characteristics, and are important in determining prognosis.
The histopathological features the pathologist looks for are the number of Reed-Sternberg cells, as well as the number of lymphocytes present in the biopsy specimen. A Reed-Sternberg cell is a funny shaped cell with two nuclei that looks like an "owl’s eyes" (see image to the right). They are believed to form when two cells merge together under the influence of certain proteins produced by the Epstein-Barr virus.
The first category, and most common type, is nodular sclerosing Hodgkin’s lymphoma. In this type, there are very few Reed-Sternberg cells with a moderate number of lymphocytes. It commonly occurs in younger individuals, and with treatment, the prognosis is excellent.
The second category is mixed cellularity Hodgkin’s lymphoma. This type has many Reed-Sternberg cells, and a moderate number of lymphocytes when viewed under the microscope. It has an intermediate prognosis.
The third category is lymphocyte predominant Hodgkin’s. It has very few Reed-Sternberg cells and many lymphocytes. It occurs most commonly in males less than 35 years of age. It is also one of the few types that is not associated with Epstein-Barr virus infection.
The last category is lymphocyte depleted. It is the rarest form of Hodgkin’s lymphoma. It typically affects older males.
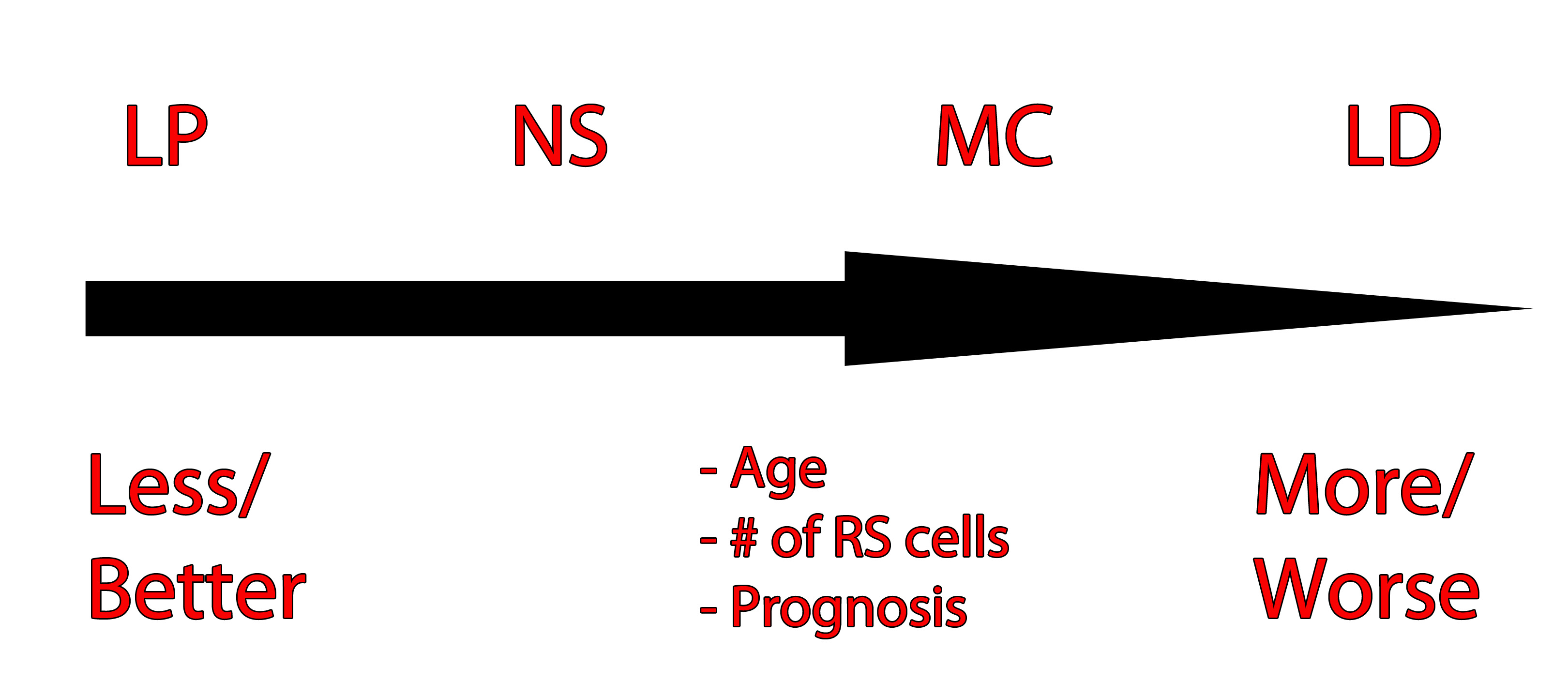
The image to the left is one way of organizing the different Hodgkin’s types and their prognosis based on age, number of RS cells, and prognosis. LP = lymphocyte predominant, NS = nodular sclerosing, MC = mixed cellularity, LD = lymphocyte depleted.
Signs and Symptoms
The classic presentation of Hodgkin’s lymphoma is painless enlargement of the lymph nodes. This is similar to non-Hodgkin’s lymphoma, and the only way to differentiate the two is through biopsy.
Systemic manifestations may occur and include night sweats, fever, and weight loss. However, these are more common in patients with disseminated (ie: metastatic) disease. Interestingly, a pathognomonic (ie: seen exclusively in Hodgkin’s lymphoma) feature that occurs in some cases is pain of the involved nodes after drinking alcohol. Finally, a symptom known as Pel-Ebstein fevers are also specific for the disease. A Pel-Ebstein fever is a cyclical fever that occurs for several weeks at a time followed by a fever free period.
Other signs related to the immune system can be seen in patients with Hodgkin’s lymphoma. A condition known as cutaneous anergy can occur. Anergy refers to a lack of response by the cell mediated immune system. For example, in patients with tuberculosis a reaction will occur underneath the skin when they get a TB test. This reaction is the result of their cell mediated immunity reacting to the tuberculosis components injected underneath the skin. However, in anergic patients no reaction is seen, even if they have tuberculosis! This can also occur in patients with Hodgkin’s disease.
Diagnosis
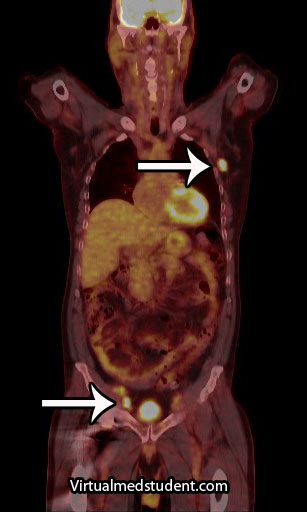
Additional studies are often performed to determine the number and location of involved lymph nodes. One such study is a positron emission tomograph (PET) combined with a CT scan. Any lymph nodes involved "light up" on the scan. An example, with the arrows pointing to involved nodes, is shown to the right.
Staging
Staging of Hodgkin’s lymphoma was traditionally based on a system known as the Ann Arbor Classification. It is divided into four stages. In stage 1 disease a single lymph node, or single organ is involved. In stage 2 disease involvement of multiple (two or more) lymph node regions on the same side of the diaphragm is present. In stage 3 disease involvement of nodes on both sides of the diaphragm is present; the spleen or other limited organ involvement may also be present. In stage 4 disease multiple organs are involved; interestingly, lymph node involvement is not necessary for a stage 4 diagnosis, although it is commonly present. Finally, each stage is further divided into “A” and “B” depending on whether or not symptoms are present. If symptoms are present, the stage is upgraded to a “B”.
| Ann Arbor Classification (simplified) | |
| Stage 1 | Single lymph node or organ |
| Stage 2 | Multiple lymph nodes on same side of diaphragm |
| Stage 3 | Lymph nodes on both sides of diaphragm |
| Stage 4 | Multiple organs involved |
However, more recently the Lugano staging classification has become the preferred method. It divides disease into limited and advanced. Limited disease includes stage 1 and stage 2. Stage 1 involves a single lymph node or nearby nodes. Stage 2 involves two or more nodal groups. Advanced disease includes stages 3 and 4. In stage 3 disease nodes on both sides of the diaphragm are involved as is the spleen. Stage 4 disease is disseminated disease into organs. There are sub-categories in the Lugano model as well.
Treatment
Like most cancers treatment is highly dependent on the stage of the disease. In most cases chemotherapy and radiation are used. Radiation is directed at involved lymph nodes, as well as lymph nodes that are uninvolved, but nearby. Common chemotherapeutic agents used include: adriamycin, bleomycin, vinblastine, vincristine, prednisone, procarbazine, and mechlorethamine.
Overview
Hodgkin’s lymphoma is a cancer of a type of white blood cell known as a B-cell. There are numerous categories depending on its histopathological characteristics. Patients often have painless enlarged lymph nodes. Some patients have fever, weight loss, and other non-specific symptoms. Staging is based on the Ann-Arbor model. Treatment usually involves a combination of chemotherapy and radiation.
Related Articles
References and Resources
- Herbst C, Engert A. Meta-analyses of early-stage hodgkin lymphoma. Acta Haematol. 2011;125(1-2):32-8. Epub 2010 Dec 8.
- Josting A. Prognostic factors in Hodgkin lymphoma. Expert Rev Hematol. 2010 Oct;3(5):583-92.
- Kumar V, Abbas AK, Fausto N. Robbins and Cotran Pathologic Basis of Disease
. Seventh Edition. Philadelphia: Elsevier Saunders, 2004.
- Armitage JO. Early-stage Hodgkin’s lymphoma. N Engl J Med. 2010 Aug 12;363(7):653-62.
- Gocheva L. Radiation therapy in Hodgkin’s disease – decades of steady progress. J BUON. 2010 Apr-Jun;15(2):226-34.