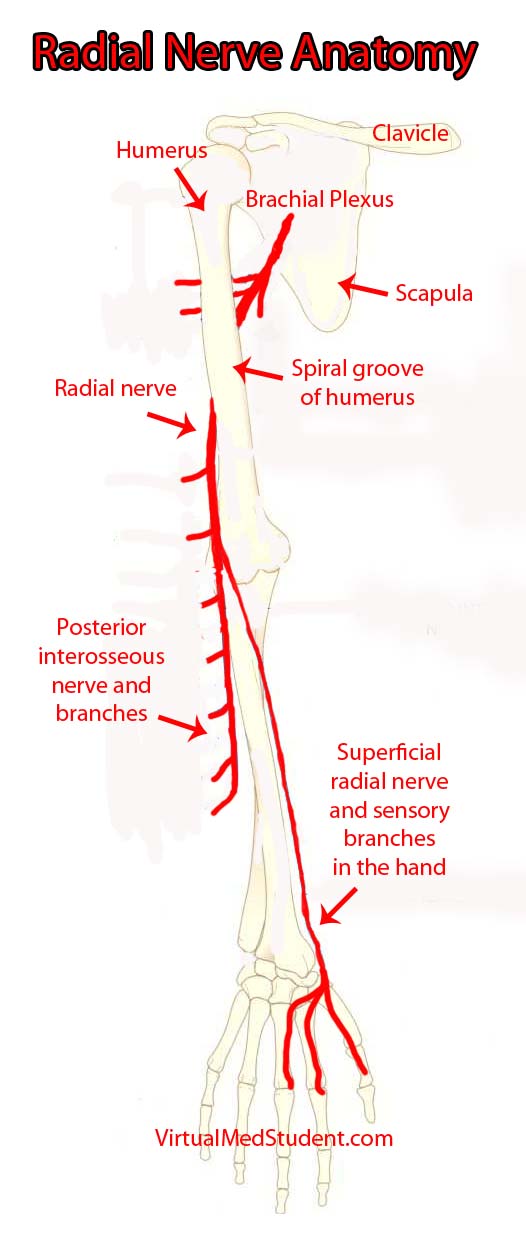
The "highways" merging into the brachial plexus are the 5th, 6th, 7th, and 8th cervical nerve roots, as well as the 1st thoracic nerve root. These nerve "highways" tangle together to form trunks, divisions, cords, and then branches. The radial nerve is one of the branches of the brachial plexus; it gets its input from the 5th, 6th, 7th and 8th cervical nerve roots.
The radial nerve courses along the humerus in the upper arm. It wraps around the humerus in a spot called the spiral groove. Just before wrapping around the humerus, it sends a branch that innervates the triceps muscle (long, medial, and lateral heads) in the upper arm.
After wrapping around the spiral groove, it sends additional branches to the brachioradialis, extensor carpi radialis longus, and extensor carpi radialis brevis muscles.
The first major branch in the forearm is known as the superficial radial nerve. This nerve courses along the medial/ulnar aspect of the forearm (ie: the ulnar or medial side of the forearm is closest to your body when your palms are facing forward) and heads straight for the hand. It relays sensory information from the lateral portion of the back of the hand.
The second major branch at the elbow can be thought of as the deep radial nerve, but it is formally known as the posterior interosseous nerve.
The posterior interosseus nerve sends branches to eight muscles in the forearm. They include the supinator (through which the nerve travels via a fibrous tunnel known as the arcade of Frohse), extensor digiti minimi, extensor carpi ulnaris, extensor digitorum, abductor pollicis longus, extensor pollicis longus, extensor pollicis brevis, and extensor indicis.
| Muscles Innervated by the Radial Nerve and Its Branches | |
| Muscle | Action of Muscle |
| Triceps brachii | – Extension of the forearm away from upper arm |
| Brachioradialis | – Helps flex the forearm closer to the upper arm – Helps supinate (ie: palm towards the sky) – Helps pronate (ie: palm towards the floor) |
| Extensor carpi radialis (longus and brevis) |
– Helps extend the wrist – Abducts the hand (ie: hand moves away from the body when the palms are facing forward) |
| Extensor digiti minimi | – Helps extend the little finger (5th digit) |
| Extensor carpi ulnaris | – Helps extend the wrist – Adducts the hand (ie: hand moves towards the body when the palms are facing forward) |
| Supinator | – Allows palms to face up towards the sky |
| Extensor digitorum | – Helps with extension of the fingers (specifically at the metacarpophalngeal joint) |
| Abductor pollicis longus | – Helps abduct the thumb |
| Extensor pollicis (longus and brevis) |
– Help extend the thumb |
| Extensor indicis | – Helps extend the index finger |
Generally speaking, the radial nerve and its branches are involved in muscles that allow joints to extend (ie: widen or separate away from one another).
Importance in Disease
Damage to the radial nerve may take the form of compression or sheering injuries, typically after traumatic events.
The most common site of injury is at the spiral groove of the humerus. The nerve may be damaged if someone breaks their humerus, or if someone leans the back of their arm on something for an extended period of time (ie: a "Saturday night palsy" is the informal term given to a drunk who falls asleep with their arms draped over a chair… they end up waking up with a radial nerve palsy!).
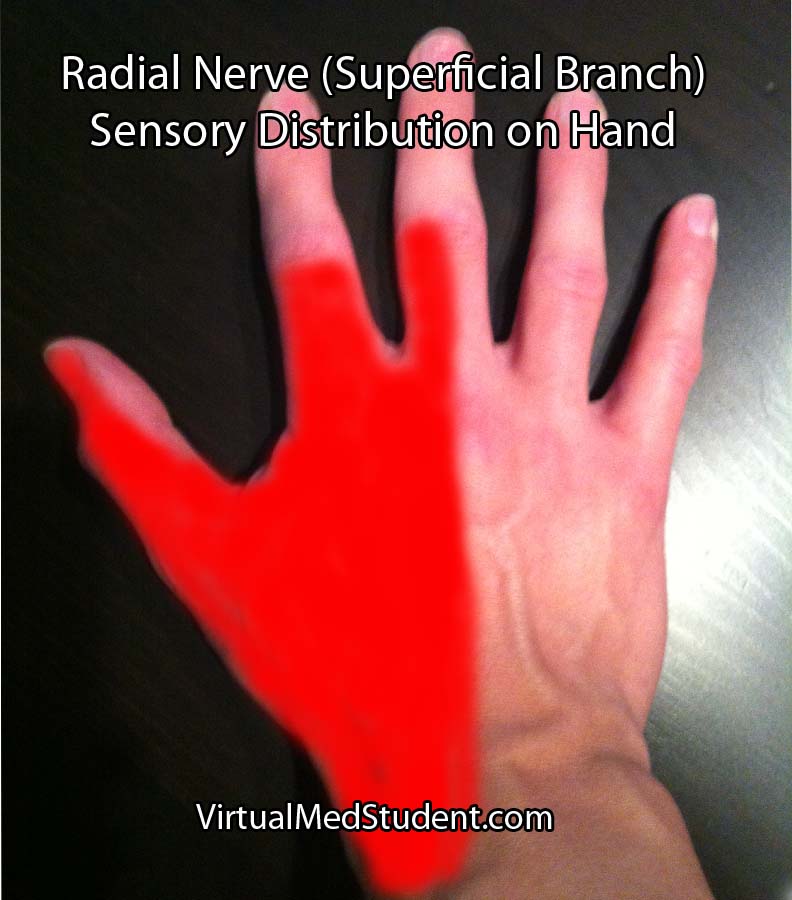
Injury to the nerve at the spiral groove causes a wrist drop, in which the affected person cannot extend their wrist. The triceps are not affected because nerve branches to this muscle are proximal to the spiral groove. In addition, patients also complain of decreased sensation on the back of the hand (see image to right).
The compression occurs at a fibrous portion of the supinator muscle known as the "arcade of Frohse", which causes an inability to extend the fingers at the metacarpophalangeal joints; this is due to dysfunction of the extensor digitorum muscle (extension at the interphalangeal – both distal and proximal – joints is controlled by the lumbricals and interossei muscles which are innervated by the median and ulnar nerves).
In posterior interosseous nerve syndrome it is still possible to extend the wrist because the branches of the nerve to the extensor carpi radialis muscle are unaffected (ie: they branch before the arcade). However, when the wrist is extended it deviates towards the radial side of the forearm because of the unopposed action of the extensor carpi radialis muscles (in other words, the extensor carpi ulnaris is affected and cannot keep the wrist extension neutral).
Sensation is entirely normal when the posterior interosseous nerve gets compressed because it contains no sensory fibers.
Overview
The radial nerve is a terminal branch of the brachial plexus. It sends branches to most of the extensor muscles of the arm and forearm. It also provides sensation to the back side of the hand. If injured it causes either a radial nerve palsy, or posterior interosseous syndrome, in which the affected patient has an inability to extend various joints at the wrist and/or fingers.
Related Anatomy You Should Know About…
References and Resources
- Ducic I, Felder JM 3rd, Quadri HS. Common nerve decompressions of the upper extremity: reliable exposure using shorter incisions. Ann Plast Surg. 2012 Jun;68(6):606-9
- Colbert SH, Mackinnon SE. Nerve compressions in the upper extremity. Mo Med. 2008 Nov-Dec;105(6):527-35.
- Reddy MP. Peripheral nerve entrapment syndromes. Am Fam Physician. 1983 Nov;28(5):133-43.
- Calfee RP, Wilson JM, Wong AH. Variations in the anatomic relations of the posterior interosseous nerve associated with proximal forearm trauma. J Bone Joint Surg Am. 2011 Jan 5;93(1):81-90.
- Arle JE, Zager EL. Surgical treatment of common entrapment neuropathies in the upper limbs. Muscle Nerve. 2000 Aug;23(8):1160-74.